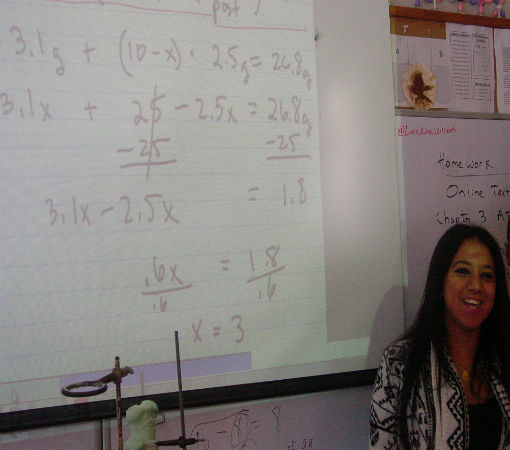Tweet to @PeriodicTableDa Book Link
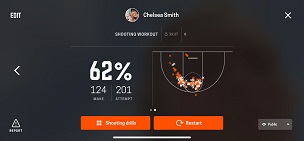
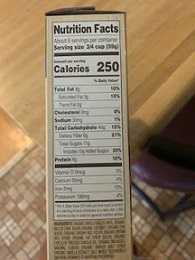
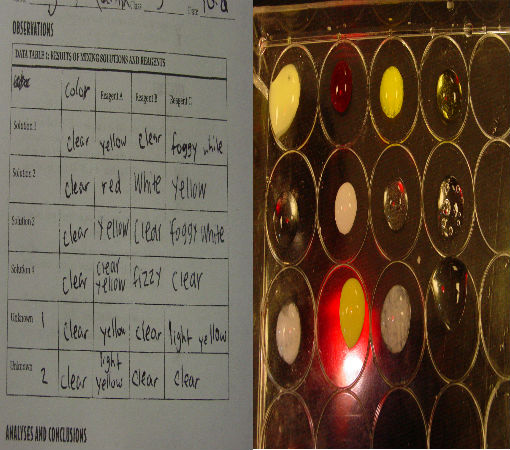
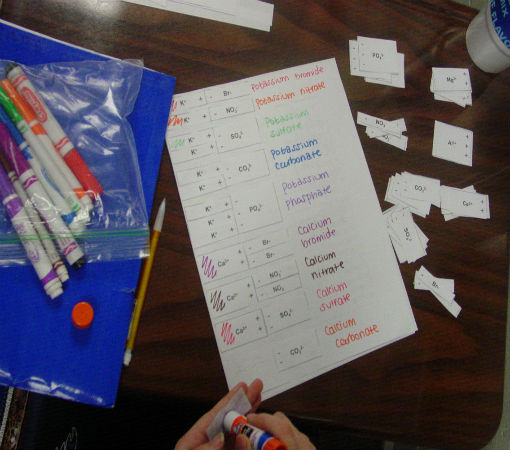
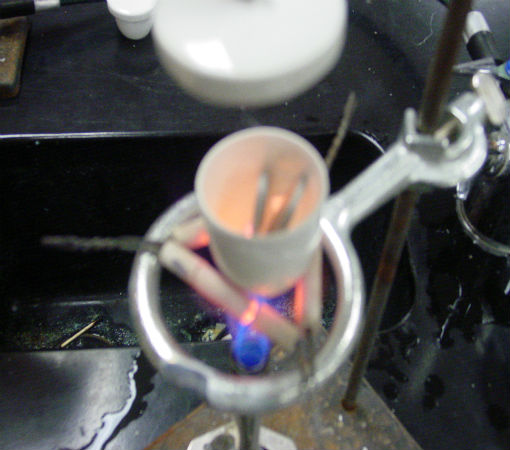
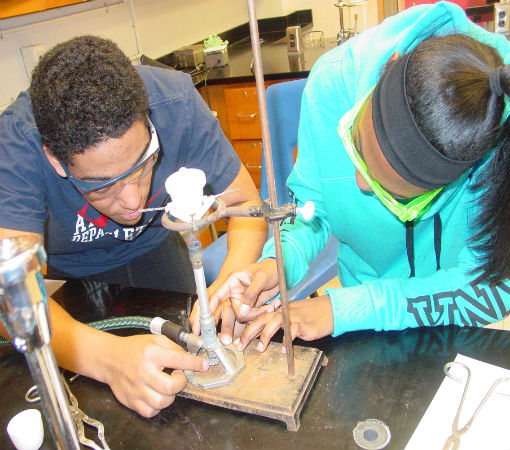
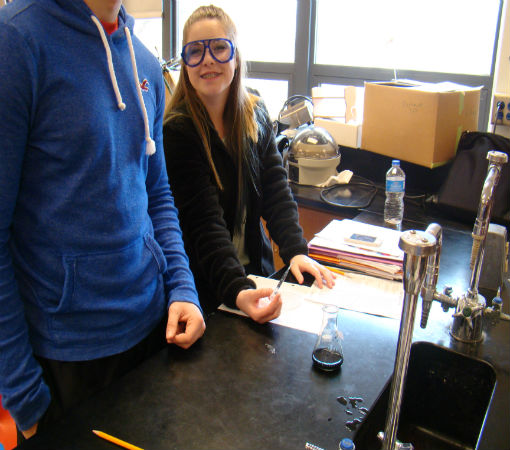
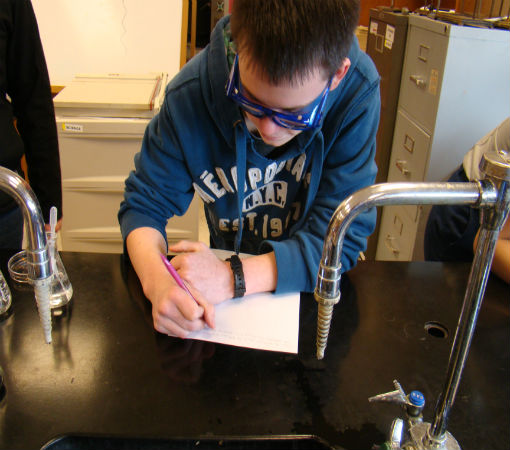
Science of Cooking: Scientific Method
|
|
|
|---|---|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Social Injustice: Persuasive writing
|
|
|---|---|
|
|
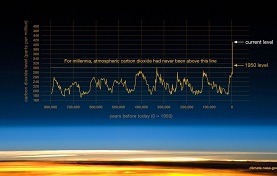
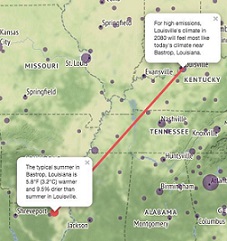
Climate Change: Persuasive writing
|
|
|---|---|
|
|
|
|
|
|
|
|
Pandemic Origin: Persuasive writing
|
|
|---|---|
|
|
|
|
|
|
|
|
Pandemic: Narrative Writing
|
|
|---|---|
|
|
|
|
|
|
|
|
Staying Healthy @ Home Descriptive Writing
|
|
|---|---|
|
|
|
|
|
|
|
|
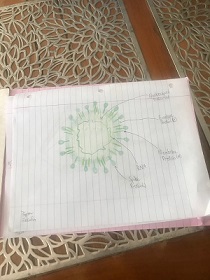
NTI: Sars-cov-2 / Corona-19 virus Expository writing
|
|
|---|---|
|
|
|
|
|
|
|
|
|
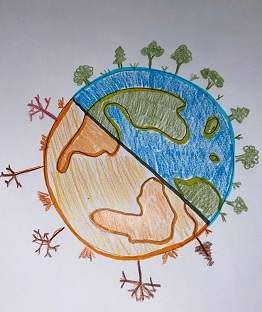
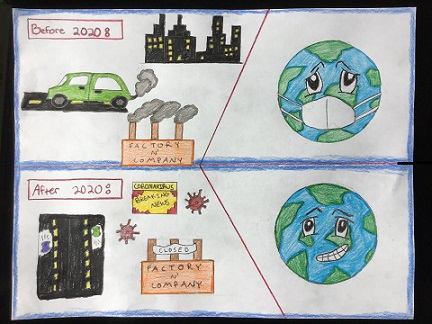
My cartoon focused on the current coronavirus situation and how it has affected the Earth. |